Standing on the shoulders of giants: Many of these ideas were incubated in the Ishwar K. Puri Lab (McMaster University, Canada), Adrian Ranga Lab (KU Leuven, Belgium), and Giulio Superti-Furga Lab (CeMM, Austria).
Engineering platforms to measure and stimulate tissue mechanics
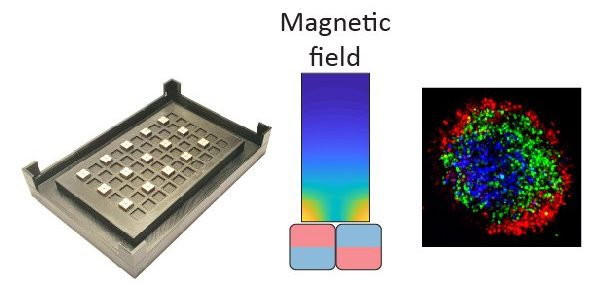
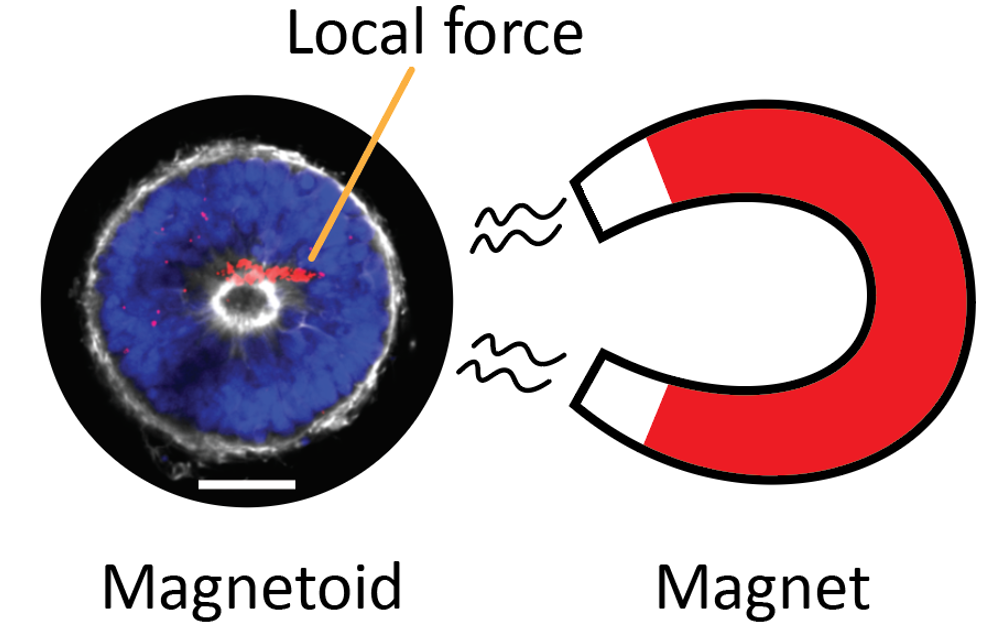
Throughout all stages of development and well into adulthood, mechanical forces play a pivotal role in orchestrating tissue shape and function. These forces, imposed on tissues by various sources, create non-uniform and transient stress fields. In our engineering unit, we focus on developing mechanical stimulation approaches to replicate such fields in both physiological and disease conditions, such as trauma and fibrosis. Using computer-aided design software, we design our tools; 3D printing enables fabrication, and programmable machines provide precise control over our devices. Our focus is on applying local active forces, where we can control both the type and magnitude of the force. This approach allows us to stimulate specific cell subpopulations and observe how forces propagate, influencing neighboring unstimulated cells. Our devices include actuator-driven rams for localized cell or organoid compression and stretching devices. However, we specialize in magnetic actuation. This innovative method enables remote stimulation of cell subpopulations (without a physical connection to a machine) and internal stimulation (within tissues using embedded magnetic particles). We are particularly excited about using this approach to measure local mechanics through particle recoil experiments. Additionally, magnetic particles can be functionalized with specialized cargo, allowing us to deliver materials to specific cells for dual mechanical-chemical activation. This expands our stimulation repertoire significantly. Our enthusiasm for magnetism extends to developing and refining a nozzle-free 3D bioprinting approach to control cellular and extracellular matrix (ECM) organization. This technique aims to create new methods for assembling 3D tissue (Cell + ECM) constructs in predefined patterns to help with tissue engineering applications and drug discovery.
Magnetic control of tissue organization
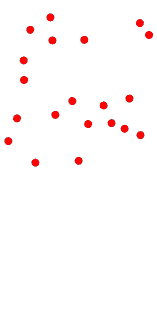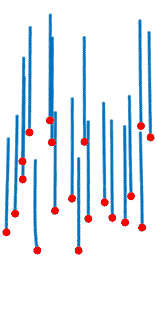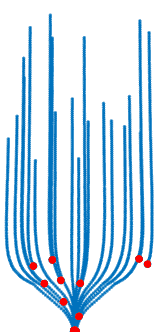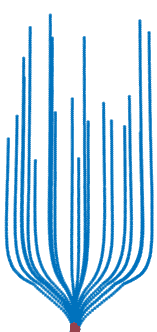
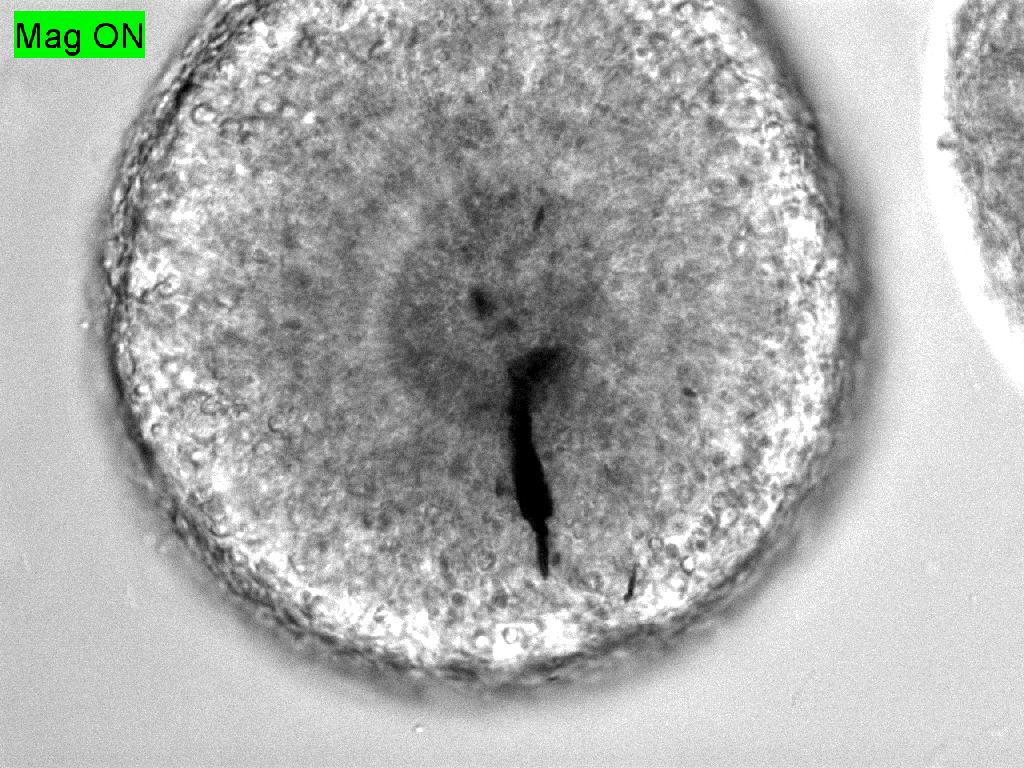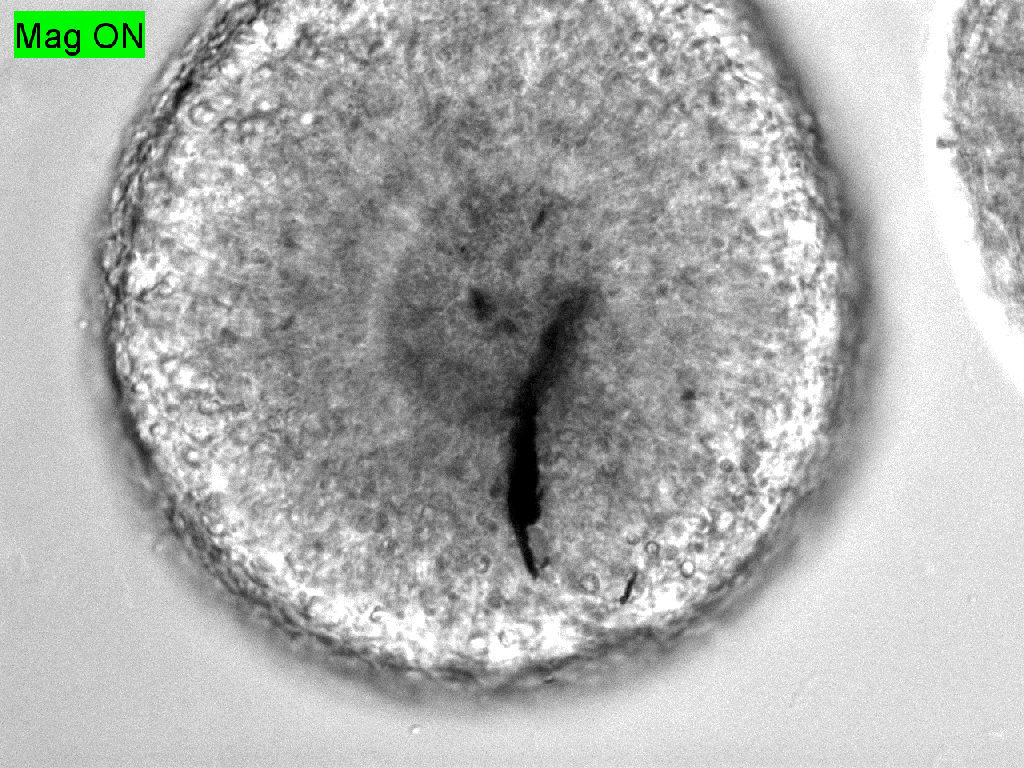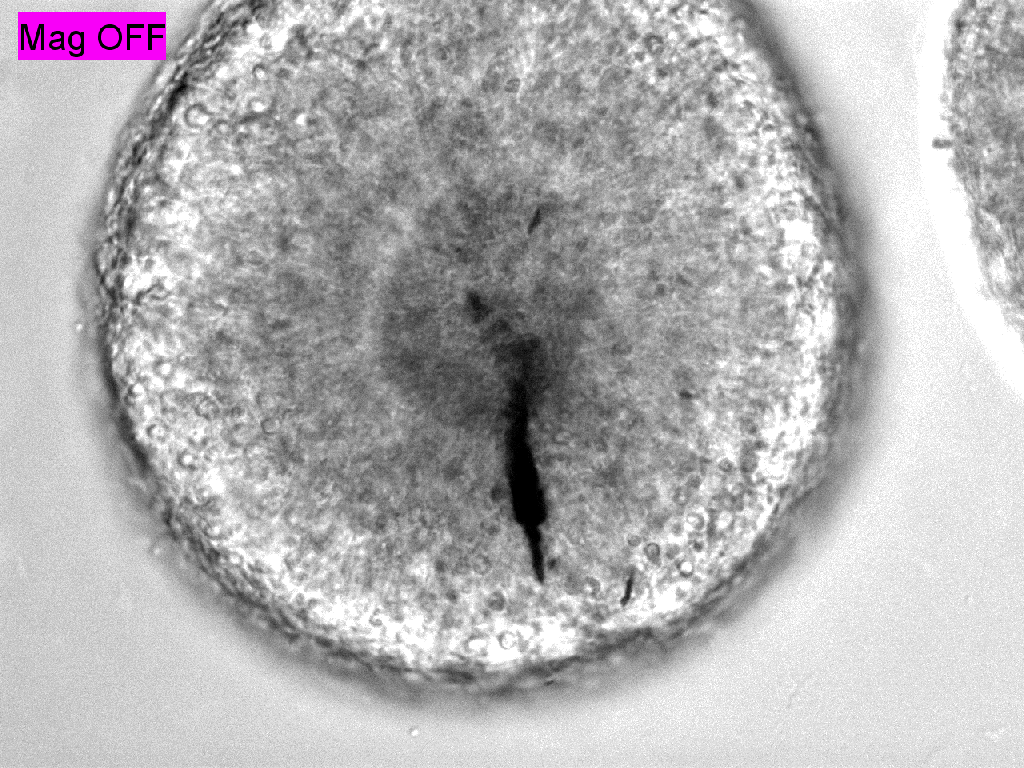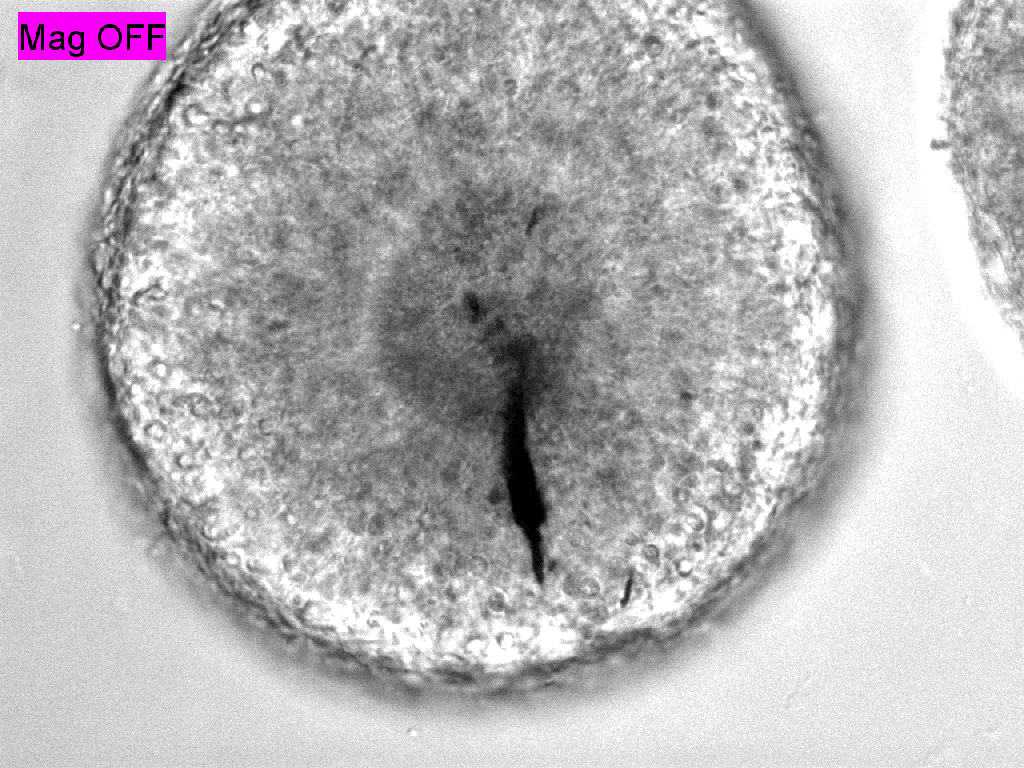
Trauma induction in organoids
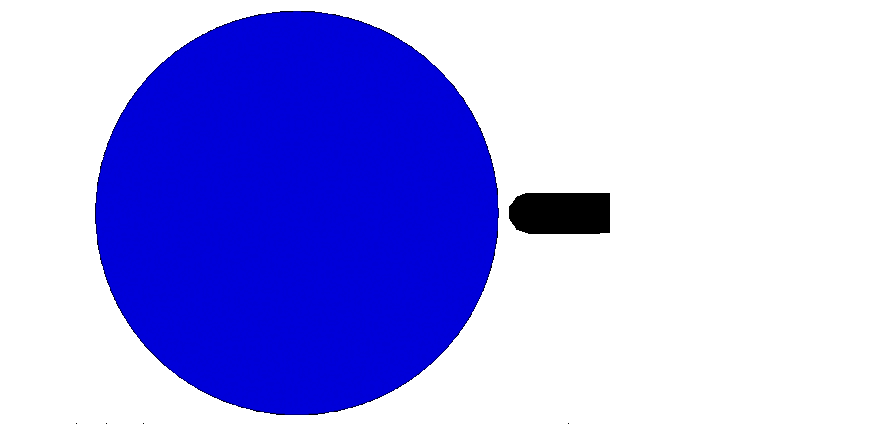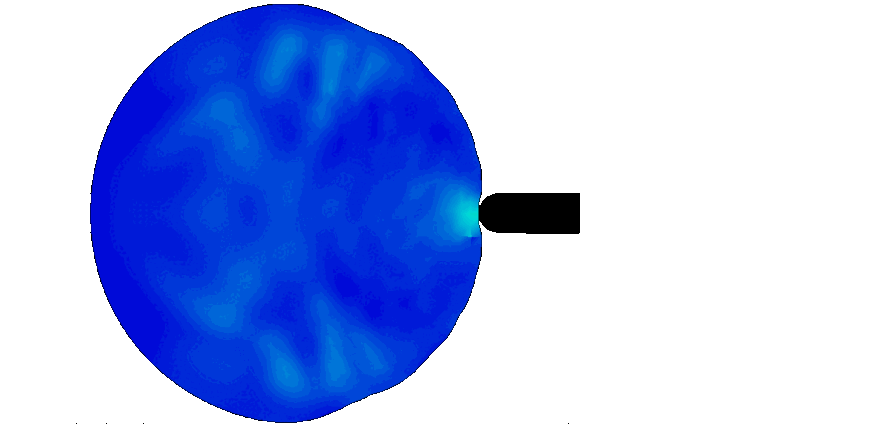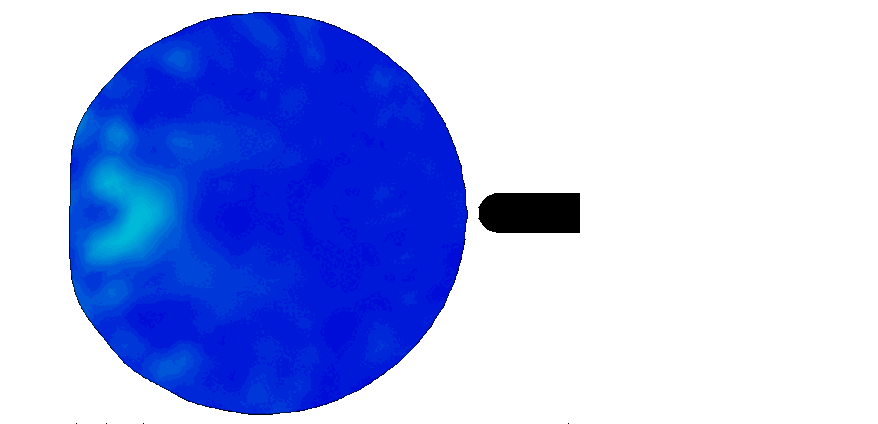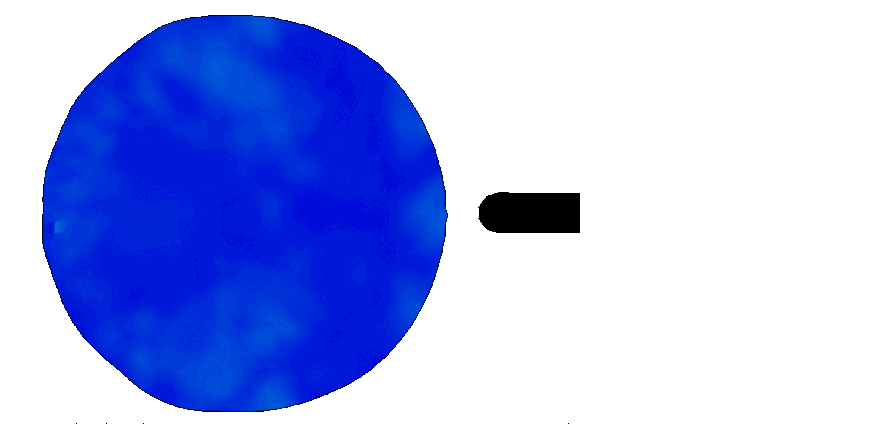
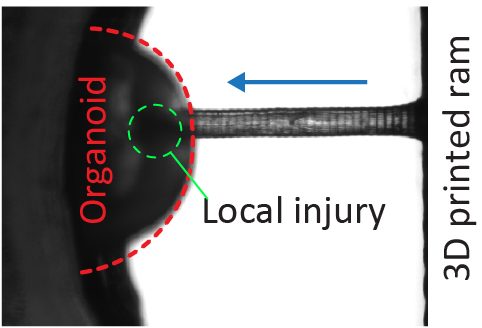
Local magnetic actuation
Mechanics of tissue organization
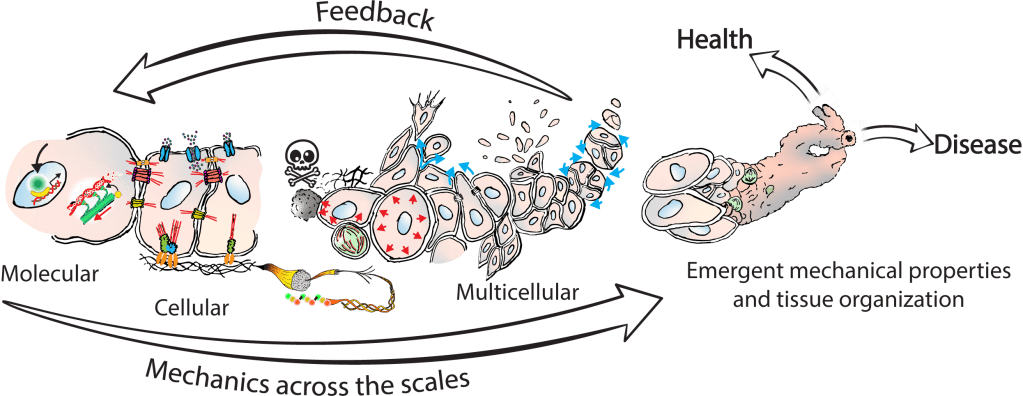
Tissues acquire specific functions through their organization. This involves ensuring that the correct cell fates are located in the right places (patterning) and that tissues adopt appropriate shapes (morphologies). Organization emerges and is maintained through coordination between morphology and cell fate specification. This relationship is dynamic, evolves over time, and influenced by various factors, such as morphogen signaling and molecular landscapes. Our primary interest lies in the mechanical aspects of tissue organization—specifically, how groups of cells use mechanical cues from their environment to initiate and maintain organization at the multicellular level, and what are the molecular drivers underlying these processes. We focus on the relationship between cells and the extracellular matrix (ECM), particularly the dynamic interactions within this meshwork. We want to understand why cells of different fates interpret the same environment differently and produce distinct morphological solutions? How do cells of different mechanical interpretation capacities coordinate/cooperate to maintain organization? Is the cell-ECM relation affected by mechanical forces and how does this play a role in organization stability? To investigate this, we use our engineered tools to stimulate and perturb in vitro model systems of tissue organization. What is our biological context? It’s diverse. We use human pluripotent stem cell (hPSCs) derived model systems, focusing on neural and mesoderm lineages and the integration of vascular networks. We investigate these questions in healthy conditions first to gain fundamental insights into the laws of tissue organization, but what happens when forces are too much to handle? We turn to our trauma and fibrosis models to study mechanics-driven tissue disorganization/reorganization. We extensively use reporter cell-lines, FRET sensors and fluorescent ECMs to report organization dynamics, which we spatiotemporally correlate to mechanical inputs through neighborhood analysis approaches. Transcriptomic analyses help us relate multicellular events to the molecular landscape, while metabolomics analyses reveal the metabolic cost of maintaining organization, quantified through entropy calculations. Finally we aim to incorporate atomic force microscopy to gain a better understanding of the cellular and ECM mechanical properties, which we can later correlate to emergent transcriptomic, cell fate and morphological domains. Through combinatorial investigations we explore the parameter space of tissue organization in various mechanical and biological conditions.
Cell-ECM coordinated tissue organization
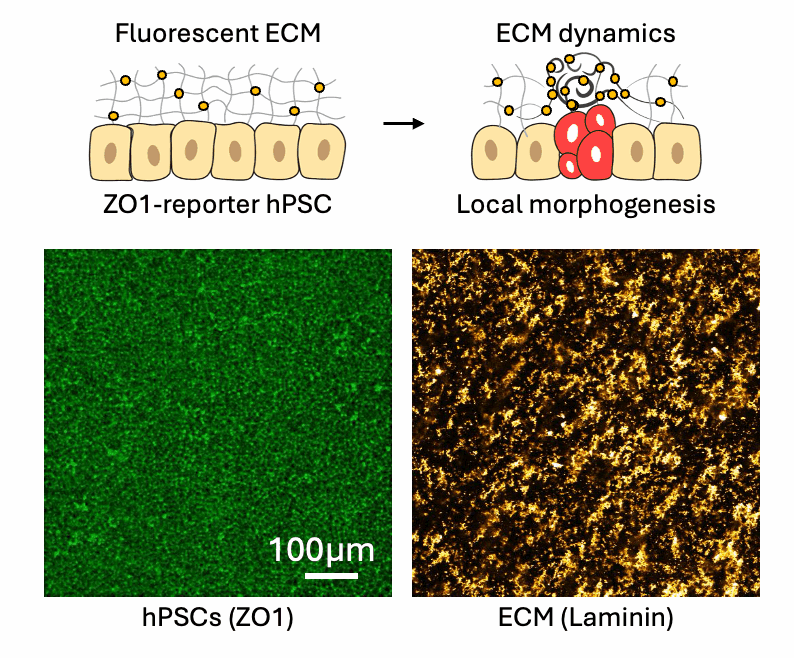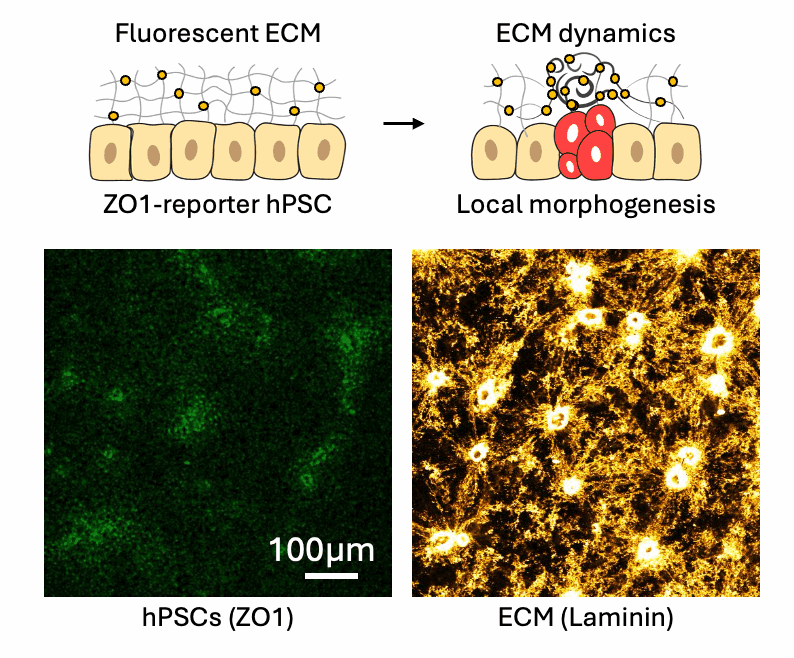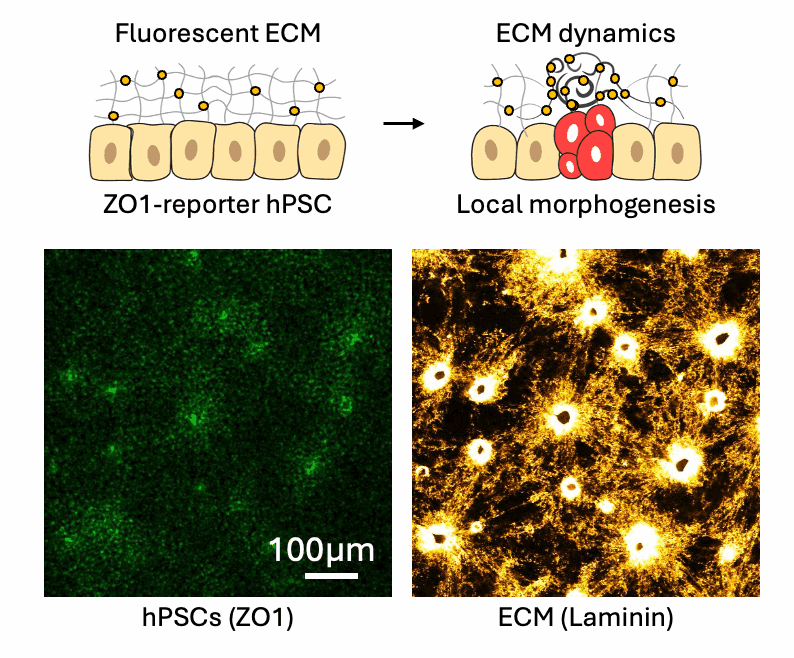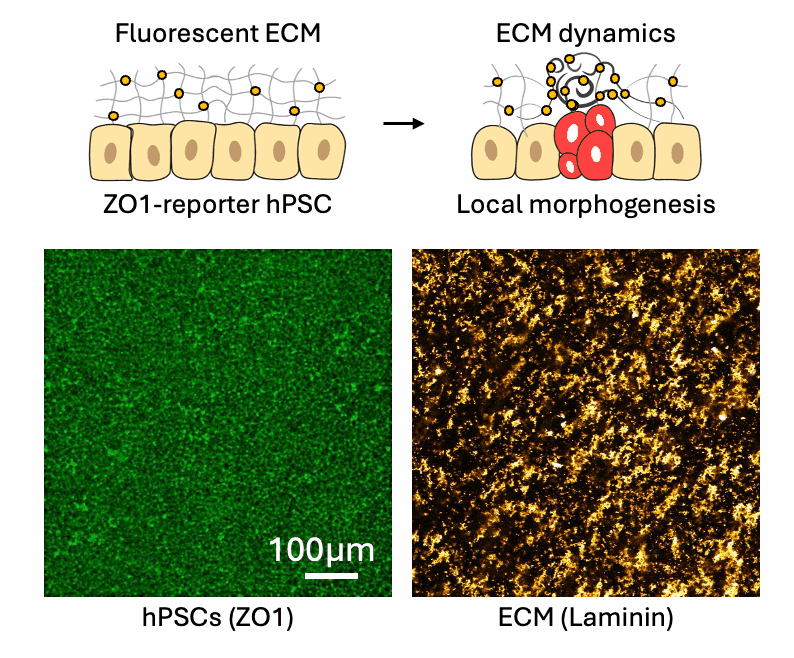
Cell-ECM neighborhood analysis

Modeling organization
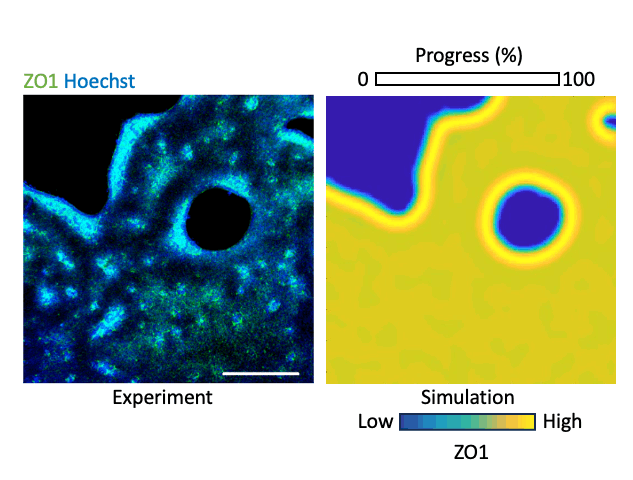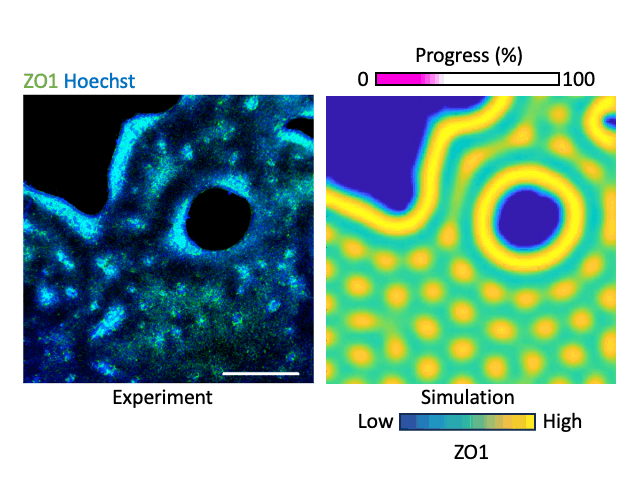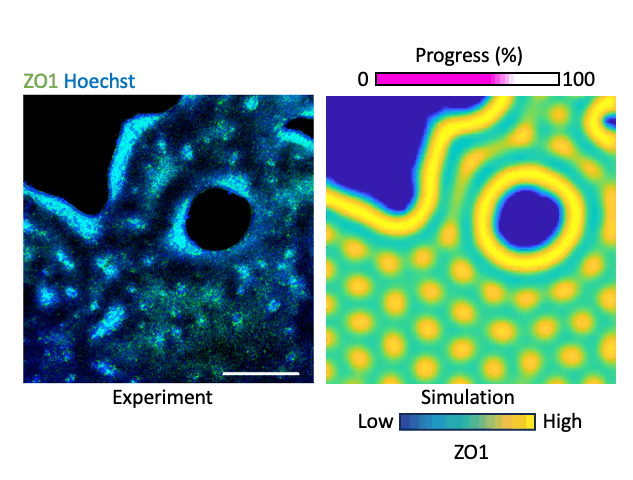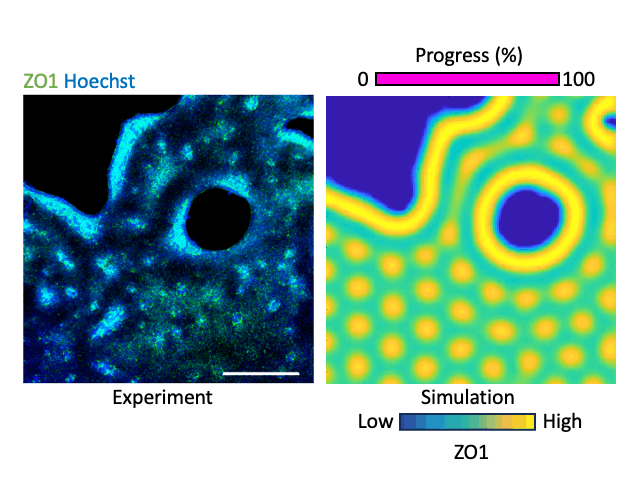
What laws govern mechanics-driven tissue organization? Using reaction diffusion and positional information-based models we investigate the physical rules that govern local interactions and lead to global organization. We work with human-interpretable 2-D models representing organizing epithelia. The models become crucial prediction tools that help us forecast the effect of perturbation, e.g. relaxing the cytoskeleton or inhibiting a matrix metalloproteinases, on tissue organization which we validate experimentally. By integrating transcriptomics and mechanical data we add layers of information to our multimodal understanding of tissue organization. Our aim is to conceive a map where organization phenotypes can be interlinked through trajectories, which are defined by the laws of tissue organization that we define. By mapping hundreds of conditions, of health and disease, we believe we can trace the roadmap of tissue organization, identify trajectories that lead to disease, and possibly trajectories that lead back to healthy conditions.
Modeling tissue organization
Want to develop the future tools and models to map the mechanical roadmap of tissue organization in health and disease? Come join us!